Plant oils have always been used as raw materials of paints and coatings. All products made from fossil fuel have a high negative impact on the environment and human health, therefore there is an increasing usage of materials derived from plant oils in the painting and polymer industry. Many plant oil based materials were developed and customized for various applications and fields.
Oxidative-polymerization (drying)
Plant oils are mixtures of compounds and the polymerization can’t be described from a simple and uniform reaction scheme. Normally the double bonds in the triglyceride chain and their chain reaction are responsible for the conversion of oil from liquid to the solid elastometric-plastometric shape.
There are different kinds of reactions that determine different types of bonds. During the polymerization besides the formation of the polymeric chains and the three-dimensional reticulum there are also competitive reactions that generate polymers and molecules with low-molecular weight (oligomers, trimers, dimers and monomers).
This phase implies the breaking of the hydrogen in the α position in correspondence to the double bond of the fatty acid chain (RH), the oxygen coordination and the hydroperoxide synthesis:
RH + O2 → ROOH
The activation of this reaction requires an energy between 35 and 65 Kcal/mole and cannot occur under ambient temperature. The reaction between oxygen and triglycerides must be regulated by external agents like heat, ionizing radiations, UV arrays, enzymes and other chemical reagents.
The most studied lipid oxidation processes occur naturally in the biological processes of food substances degradation and in the metabolism of living beings: the Photo-Oxidation (UV arrays) and the lipoxygenase (enzymes and metal proteins).
The study of the reactions between oxygen and lipids has deep roots, even Lavoisier during the French revolution was already interested in the burning and oxidation of fats and oils at low temperatures, for example. These first examination can be considered as the birth of the organic chemistry.
This process involves initially a photosensitiser, which is a molecule that goes into excited state under the exposure of a light with a specific wavelength. Some examples can be dyes, pigments or for plant oils riboflavin, chlorophyll and erythrosine.
The excited substance can react directly with the substrate or a molecular oxygen starting a series of energy transfers that will eventually result in an oxygenated molecule.
Therefore, the photo-oxygenation reactions are classified in terms of order and type of this intermediates (reactions of type I,II and III):

In the first example the sensitizer reacts directly with the fatty acid and forms a free radical (R•) that reacts with the oxygen to give rise to the hydroperoxides. In the second example the excited sensitizer reacts with atmospheric oxygen (O2 in normal triplet condition) to form the more reactive O2 as a singlet. This reacts with the fatty acid and gives rise to the hydroperoxides. The singlet oxygen reacts differently with the double bond of unsaturated fats and originates a concerted addition mechanism where the oxygen enters the end of both Cs of the double bond and then moves to the allyl position with trans configuration. The resulting hydroperoxides now have a trans allyl double bond.

The lipoxygenase is an enzyme that acting on the polyunsaturated fats through oxidation reactions can synthetize the conjugated hydroperoxides.
Its reaction can occur only if there is a 1-4 cis, cis-pentadienoic group in the hydrocarbon chain.
The processes catalysed by enzymes are stereo- and regiospecific according to the involved enzyme and the oxidation products, despite what happens during a normal oxidation reaction.
This enzyme forms the pentadienoic radical thanks to an iron atom. For a subsequent reaction with the oxygen it becomes a peroxidic radical. The reaction of iron in the ferrous form with the peroxidic radical generating an anion and taking the iron back to its ferric state completes the catalytic enzymatic cycle.
The formation of a free radical establishes the breaking of the carbon-hydrogen bond. The energy needed for this operation depends on the whole molecular structure, especially from the neighbourhood near the dissociative bond.
The more bonds we have with other carbon bonds than with hydrogen bonds, the less energy will be required. For methane (CH3-H) 102 kcal are required, whereas for primary, secondary and tertiary hydrogens only 90 kcal will be sufficient. High energy values and the breaking of these bonds occur only under elevated temperatures.
Double bonds allow to reduce the energy required for the bond dissociation, for example for the propylene this value is about 78 kcal, whereas for hydrogen atoms belonging to a methylene inserted between two conjugated double bonds less than 78 kcal are required. This occurs in unsaturated fatty acids made of linoleic acid containing a methylene or two methylene groups.
The free radicals (R•, RO• e ROO•) formed during the initial oxidation phase are very reactive and many reaction mechanisms are possible for them.
The free radicals can react with one molecule of unsaturated acid extracting a hydrogen atom and giving rise to hydroperoxides generating a chain reaction (mono-molecular propagation phase).
Mono-molecular propagation phase
R• + O2 → ROO•
ROO• + RH → ROOH + R•
When the concentration of hydroperoxides exceeds an established value, a bimolecular propagation phase starts. This is regulated by a different kinetics and characterized by a high-speed reaction.
Bimolecular propagation phase
2 ROOH → ROO• + RO• + H2O
ROO• + RH → ROOH + R•
RO• + RH → ROH + R•
The mono-molecular propagation phase (one hydroperoxide) occurs only when the substrate of fatty acids has an oxidation degree of 1%. In case of higher values, occurs the bimolecular propagation phase, whose speed is proportional to the peroxides content.
High speed implies an increased concentration of alkoxides and peroxy-radicals in the fat. This is a self-sustainment phase in the radical chain reaction.
The above described reactions occur during the two propagation phases and don’t allow the formation of a triglyceride grid, which can be formed only if the peroxy-radicals are added to a double bond chain, especially if they are conjugated.
The union of peroxide groups or the alkoxide radicals originated by the homolysis of hydroperoxides and peroxides may give rise to dimers. These dimers are formed by ether bonds with a single bridge-bonded oxygen atom.
The highest level of peroxide formation marks the beginning of the termination phase.
Fats, oils and food always contain heavy metals which cannot be completely deleted even through an industrial refining process. These ions (mainly iron, copper and cobalt may come from impurities in vegetal materials, oil seeds packaging or from other equipment. Catalysing the decomposition of hydroperoxides in radicals that start new radical chains in the oxidation process, these ions are responsible for the propagation phase.
The plant oils belonging to the linoleic acid group (corn and sunflower oils) can easily oxidize and can contain less than 0.03 ppm of iron and 0.01 ppm of Copper to grant an acceptable stability.
This limit value can increase up to 5 ppm (for Copper and Iron) if the animal fatty acids have a high content of oleic and/or stearic acid.
The presence of a hydro peroxidic group is an essential requirement for the metal ion to be an oxidising catalyser and leads to the hydroperoxide decomposition and the new radical chain:
Men+ + ROOH ® Me(n+1)++ RO· + OH—
Me(n+1)+ + ROOH ® RO·2 + H+ + Men+
All radicals formed in the propagation phase can directly react in contact with double bonds and start the polymerization for addition.
In fact, the radicals ROO• e RO• can attack the double bonds of other fatty acid chains and bind to them forming a grid through peroxidic (R-O-O-R) or ethereal (R-O-R) bridge-bonds.
Bridge Bonds C-C are generated in two ways: through the recombination of two R• radicals after the attack of a R• radical to a double bond C=C if there is a low O2 concentration.
Triglycerides do not give rise to very long and linear polymeric chains as during vinyl-monomer polymerization but create a grid (ramified polymer with three-dimensional development).
The oxidative process continues with the transformation of the hydroperoxides into oxidation secondary but not radical products. The main hydroperoxide decomposition mechanism implies the splitting of the double bond next to the hydroperoxide group and leads to the formation of hydrocarbons, aldehydes, alcohols and flying ketones. Sometimes other secondary non-flying compounds (non-flying aldehydes, oxidised triacyclglycerols and their polymers) may rise from this process. The kind of splitting of the double bonds in the fatty acid chain and the hydroperoxide decomposition determine the products obtained from the lipid oxidation. The reaction may also end after the polymer formation and many antioxidants can make the termination of the radical oxidation chain easier.
An oxide-polymerization process with a substrate of single fatty acids develops mainly dimers or possibly trimers, whereas in triglycerides, since they are formed by three chains of unsaturated fatty acids united in each molecule, this process generates a grid with three-dimensional cross-links.
A triglyceride dimer formed by the union of one out of three chains of the triglyceride molecule will still contain 2+2 chains of unsaturated fatty acid and can be used for other polymerization reactions. Moreover, the union of two polymers forms a tetramer with 3+3 chains of unsaturated fatty acids available to repeat the process and then continue for other sequential and subsequent stages.
These development stages lead to the formation of a highly ramified network.
The drying reaction is originated and controlled by many factors: heat, pressure, oxygen availability, UV arrays, presence of photosensitisers (pigments, vitamins and other compounds), enzymes, metal-proteins, metal ions, antioxidants etc.
The oxidative-polymerization process is determined by a radical-free chain reaction scheme that occurs in three phases:
— Initialization phase: the reaction of the atmospheric oxygen with the most reactive site (unsaturated bonds) of the triglycerides to form very reactive species R•, RO• e ROO• .
— Propagation and termination phases: in these last two phases occur the synthesis chain reactions.
Cyclization
It is possible to carry out many transformation and modification processes of plant oils generating liquid mixtures where the triglycerides are made reacting before the application and the final use of oil.
The result of this changing leads normally to an increased kinematic viscosity and sometimes these processes can be classified as “densifications”.
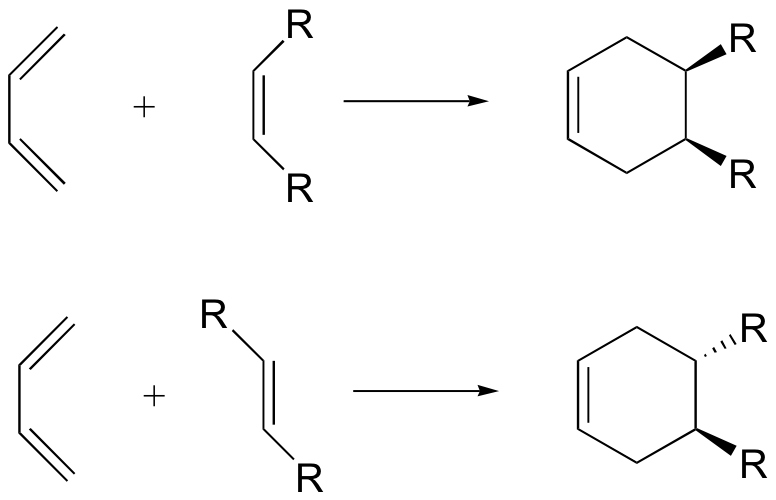
The modification reactions vary in accordance with the raw materials, reagents and process parameters (like air, temperature) composition. If there is air, these are mainly pre-reactions of controlled oxide-polymerization processes, on the contrary if there is no oxygen other reactions occur; like the Diels-Alder ones for example.
In this case the reaction scheme establishes the formation of a cyclic compound obtained through the union of a conjugated diene with a compound of a single double bond. For this reason, this compound can react with another diene and form a trimer.
The synthesis of cyclic monomeric acids can be carried out only through heating or using alkaline conditions, which help the isomerisation.
Transesterification
The transesterification of plant oils is based on the triglyceride reaction with an alcohol under the presence of an acid or basic catalyser and the production of an ester mixture of glycerol and fatty acids. A 3:1 molar relation between alcohol and triglyceride is needed to complete the reaction from a stoichiometric point of view. Since the transesterification is a balanced reaction, the excess of alcohol allows to move this reaction towards the ethers formation.
From a chemical point of view, biodiesel is made of alkyl ethers that may derive from the triglyceride transesterification or the esterification of fatty acids with alcohols with low-molecular weight (like methanol). Its physical features strongly depend on the starting material used.
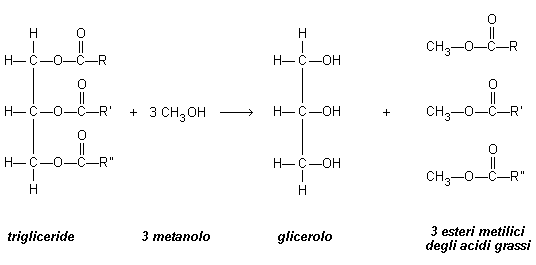
The triglycerides used as a starting material may derive from starch and sugars or other oils like canola or palm oil. In 2011 most of the biodiesel from the European Union has been produced from canola oil (67%). This value does not impact on food availability and has a beneficial effect on agriculture since canola is a circular cultivation that re-establishes the ground nourishments and balances the nitrogen cycle. Biodiesel produced from plant oils starts the production of high volumes of protein materials for animal feed. In the European Union the production of Biodiesel led to the production of 13 billion tons of proteins, which correspond to half of protein mils produced in the EU.
The transesterification of plant oils can be used for the industrial synthesis of alkyd resins.
The direct reaction of dicarboxylic acid and polyol dispersed in oil leads to a polyester reaction without involving the oil triglycerides in the reaction since the acid is insoluble in the oil and reacts only with the polyol.
Initially the resin synthesis technology through transesterification foresees the reaction of a polyol with the plant oil. In this way the compounds able to react through polycondensation can be synthetized.
This first phase of the process leads to the formation of diglycerides, triglycerides and glycerine. According to the process parameters, 250°C and an alcaline catalyser are needed to start the reaction.
The second phase occurs under a slightly lower temperature through the adding of the acid and not before the end of the reaction between the polyol and the oil. About 50-60% of the alkyd resins is made of fatty acids, glycerol and pentaerythritol and the rest of phtalic acid.
Chemical modification
Plant oils in their virgin or raw forms require long times of drying and the resulting films do not satisfy the minimal needs for the physical-mechanical performances and corrosion resistance. Therefore, many changes and chemical reactions are carried out in the functional groups inside the plant oils. The most common chemical reactions that occur in polymers are for example the epoxidation, hydroxylation, acylation and isocyanation. These reactions lead to the formation of monomers/polymers used actively as alloying materials for protection and coating equipment.
The big advantage is the property of modified plant oils to keep a low viscosity and high flexibility of the obtained coatings. As a consequence, these oils can be also used as reactive diluents or used directly as coating liquid products without using any solvent.
Some plant oils like Vernonia Euphorbia and Cepbalocroton contain a lot of vernolic acid, a fatty acid with a natural oxirane ring (epoxydic group) in the triglyceride chain. This ring can be also included in the plant oils unsaturation sites through a chemical reaction. This process is called epoxidation and can be carried out with many processes through peracids, enzymes hydrogen peroxide, dioxane, a phase-transfer catalyser etc.
Because of their long hydrophobic chains, all epoxidated plant oils have a high flexibility and resistance to corrosion especially against humidity and other chemical substances.
If there are ester groups, the molecules can be broken at the glycerol and the fatty acid level through hydroxylation to produce polyols.
Polyols are mainly obtained from non-renewable oil sources or from renewed plant oils through epoxidation and hydroxylation. Plant oils are used to introduce epoxydic groups because of the presence of unsaturated bonds in oleic, linoleic and linolenic fatty acids.
The polymeric polyols are used as reagents to produce other polymers and can be reacted with isocyanates to produce polyurethanes. This use consumes the majority of polyether polyols. Moreover this materials are used for elastometric shoe stoles, fibres, foam insulator for household appliances (fridges and freezers), adhesives, mattresses, automobile seats etc.
Polyester amides PEA are modified alkyd resins obtained through the esterification between diol amid from a plant oil with acid or anhydride. They contain repeated ester and amid units in their chain and have improved properties like easy drying, water vapor resistance and resistance to chemical substances, especially alcohols.
Polyetherimindes [PEtA] are polymers made of parts alternated with amid and ether groups and were introduced as polymers staring from plant oils in 2013. Their synthesis is a double-phase process:
— the preparation of diol amid from plant oils
— the condensation reaction between amid diols, A bisphenol [BPA] and resorcinol.
About 50% of the PEtA is bioderived and biodegradable in nature.
Other reactions
The alkaline compounds can break the esther bond, then they release glycerol and fatty acids that form salt reacting with alkaline ions. If necessary, the soaps can be precipitated jumping separately with a solution of saturated sodium chloride. Normally sodium hydroxide is used to produce hard soap whereas the potassium hydroxide for the production of soft soap.
This reaction consists in the transformation of triglycerides with chains of unsaturated fatty acids into triglycerides with saturated fatty acids. This process is obtained through the reaction with the hydrogen and is catalysed through nickel-based compounds.
An example of hydrogenation is the production of margarine, which is an alimentary fat obtained through the catalytic hydrogenation of plant oils emulsified in water. On the other hand butter is a mixture of triglycerides made of short-chain (up to 16 carbon atoms) fatty acids.
Plant and fat oils treatment with aqueous alkali (like sodium hydroxide and potassium hydroxide) leads to the formation of sodium or potassium salts of acid fats (soaps) and glycerol.
This reaction is known as saponification.
HOME > MATTER > OIL > REACTIVITY > RENEWABLE SOURCE


 Italiano
Italiano English
English



















